Ground-breaking gene therapy helps boy with congenital vision loss
- UdeMNouvelles
05/30/2023
- Béatrice St-Cyr-Leroux
For the first time in Quebec, gene therapy is being used to reverse a rare disease caused by a genetic defect, and it’s thanks to the tireless work of two Université de Montréal doctors.
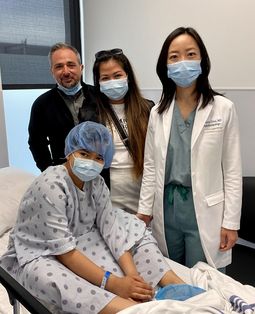
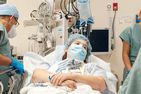
Eleven-year-old William has Leber’s congenital amaurosis, a rare hereditary disease that affects the retina and causes vision impairment and even blindness. But there’s hope for William: in May, he received an innovative new treatment that could permanently improve his vision and, if all goes well, slow or even stop the progression of the disease.
William is the first person in Quebec to receive a gene therapy designed to inhibit a disease by correcting the underlying genetic defect. In William’s case, the problem is a mutation in the RPE65 gene that prevents photoreceptors in his retina from properly converting light into electrical signals.
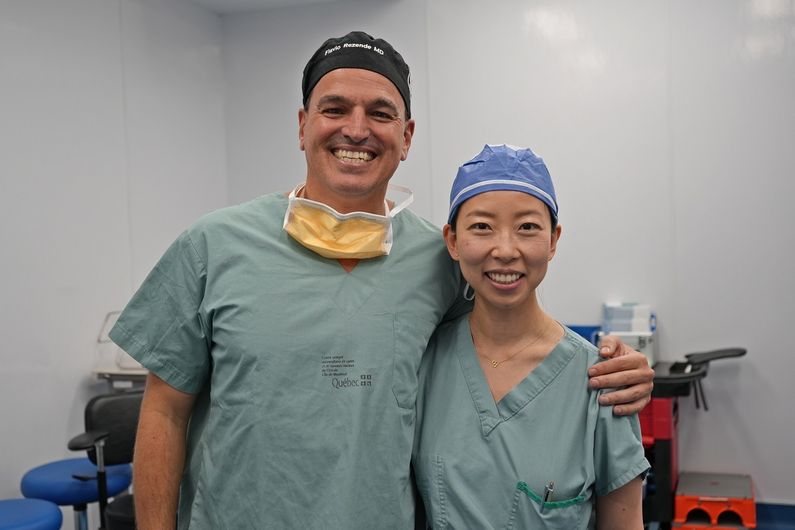
The treatment was approved by Health Canada in 2020, and then by Quebec for coverage under its public health plan in 2022. The surgery needed to administer the gene therapy was performed by Cynthia Qian and Flavio Rezende, retinal surgeons at the Ophthalmology University Centre of Maisonneuve-Rosemont Hospital and clinical professors in UdeM’s Department of Ophthalmology.
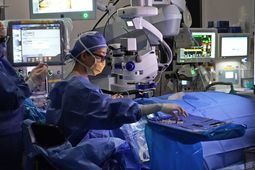
The surgery involved carefully injecting a solution—the gene therapy drug Luxturna—under the retina of William’s eye. This drug contains a healthy gene to replace the defective one. Once inside the retinal cells, the new gene can start producing the missing protein that is preventing the retina from doing its job.
Just the beginning
The surgery performed on William was the culmination of almost 30 years of research, approval processes, clinical trials and practice to perfect the technique. While the RPE65 gene was identified back in 1993, Luxturna was only first approved in 2017, by the U.S. Food and Drug Administration.
Following Canadian approval in 2020, Qian worked tirelessly with her partners at Université de Montréal, Maisonneuve-Rosemont Hospital, Ste-Justine Hospital and the McGill University Health Centre to put everything in place to make this ground-breaking surgery a success.
“For the first time, gene therapy is being used to cure a disease by replacing a defective gene with a functional gene, not just for purposes such as alleviating symptoms,” said Qian. “We hope our success will accelerate access to this treatment and also pave the way for other gene therapies to treat all sorts of genetic diseases.”
Buoyed by the success of this first procedure, Qian is convinced gene therapy will reshape the future of medicine. “This technology will not only help advance medical science,” she said, “but also, and more importantly, benefit people suffering from previously untreatable diseases.”