New eye drug may someday help diabetic patients
- Salle de presse
02/06/2024
- UdeMNouvelles
Scientists have developed an experimental medication called UBX1325, or foselutoclax, that shows promise in treating macular edema.
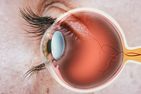
Diabetes can lead to eye problems, and a common one is diabetic macular edema (DME), causing vision loss. Now a study published in Nature Medicine suggest a new experimental drug could someday help make treatment of DME more successful and longer-lasting.
The drug – part of a new class of therapeutics called “senolytics” – was developed by scientists at San-Francisco-based UNITY Biotechnology and the Maisonneuve-Rosemont Hospital Research Centre, affiliated with Université de Montréal.
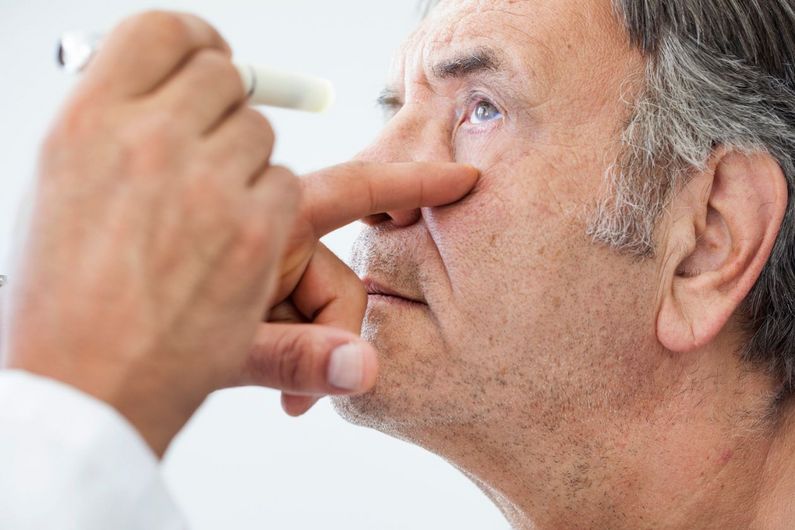
Called UBX1325, or foselutoclax, the drug was tested on diabetic patients who didn't respond well to regular treatment.
DME occurs when tiny blood vessels that supply the retina start to leak, cause swelling and vision problems. Current treatments, effective for about half of diabetic patients, often require frequent eye injections and may come with side effects.
The new drug eliminates the troublesome cells, helping the eye to heal.
"We developed UBX1325 to selectively eliminate damaged, senescent cells that propagate disease in the diabetic retina," said the study's lead author, UdeM ophthalmology professor Przemyslaw (Mike) Sapieha, the chief scientist at UNITY.
"By removing senescent cells from the vascular unit, we believe we stimulate healing of the retina," said Sapieha, who holds a Canada Research Chair in Retinal Cell Biology and UdeM's Chaire du FROUM (Fonds de recherche en ophtalmologie).
Just one shot
In the study, patients received just one shot of UBX1325, and the positive effects on their vision lasted at least six months.
"This project was initiated over six years ago, and seeing the translation of this work from bench to bedside has been very exciting," said UdeM assistant professor of optometry Sergio Crespo-Garcia, the study's first author.
"We look forward to exploring the role of cellular senescence in retinal disease beyond diabetic retinopathy."
With 93 million people globally affected by some degree of diabetic retinopathy, according to the American Academy of Ophthalmology, the prevalence of this condition is expected to rise significantly as diabetes continues to spread worldwide.
For over a decade now, the UdeM scientists have worked hard with UNITY to identify molecular pathways that can be targeted to selectively eliminate senescent cells while sparing healthy ones.
UNITY Biotechnology is currently advancing UBX1325 in the Phase 2b ASPIRE trial, a randomized, double-masked, active-controlled study. Data from this trial is expected in the fourth quarter of 2024.
About this study
"Therapeutic targeting of cellular senescence in diabetic macular edema: preclinical and phase 1 trial results," by Mike Sapieha, Serio Crespo-Garcia et al., was published Feb. 6, 2024 in Nature Medicine.
Media contact
-
Jeff Heinrich
Université de Montréal
Tel: 514 343-7593